APOLLO-B
On August 3, 2022, Alnylam announced that its siRNA drug patisiran had achieved positive results in the phase III clinical trial APOLLO-B, which significantly improved the symptoms of patients with transthyretin amyloidosis cardiomyopathy (ATTR-CM), and improved the 6-minute walk test index (primary endpoint, p = 0.0162) and quality of life (one of the secondary endpoints, KCCQ score, p = 0.0397). At the same time, the patisiran also demonstrated good safety.
transthyretin (TTR) is mainly produced by the liver. In ATTR patients, due to gene mutation or acquired reasons, TTR protein deposits into fibers in multiple organs, affecting the normal function of organs. The organs and tissues affected by ATTR include heart, digestive tract, urinary system, nervous system, etc. Among them, ATTR-CM is the most harmful and usually leads to heart failure.
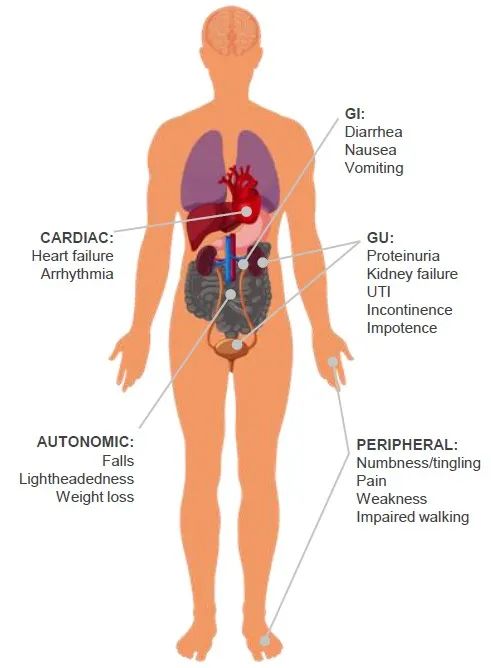
Figure 1. Organ and Induced Symptoms Affected by Transthyretin Amyloidosis (ATTR Amyloidosis) (Source: 3)
Patisiran are siRNA drugs that target TTR mRNA degradation by intravenous injection and carried by lipid nanoparticles (LNP). Patisiran is not a new drug. In fact, it is the first drug to be marketed in Alnylam (trade name: Onpattro) and the first approved siRNA drug in the world. As early as August 2018, it was approved by FDA to treat hereditary ATTR with polyneuropathy (hATTR-PN). However, compared with the hATTR-PN market (2-30000 patients per year worldwide), the ATTR-CM market is much larger (20-300000 patients per year worldwide).
this opportunity, we conducted an interview with the RNA targeted therapy company SynerK to explore the significance of APOLLO-B success and the development of RNAi drugs.
RNA Targeted Therapy Company SynerK
SynerK was founded by several senior experts who have long worked in the RNA therapy industry and has research and development bases in Suzhou, Beijing and Boston. Shi Nengkang has a very complete and complementary core team in the field of RNA therapy, including senior biomedical experts with rich successful experience in the field of RNA therapy from research and development to patent medicine. Team members have also participated in the work related to a number of successful siRNA drugs. The team's rich experience and expertise cover all major aspects of RNA drugs from target selection to delivery technology, from research and development to marketing.

Schnengkang has innovative delivery technology targeting liver and extrahepatic organs. The company selects targets according to its unique procedures to expand the treatment pipeline, not limited to the fixed treatment field, with its own technology platform as the core, to establish a variety of different indications of the pipeline. The first of these drug candidates is expected to enter the clinical phase in 2023. The company's vision is to become the world's leading biopharmaceutical company for targeted RNA therapy, benefiting patients with a variety of RNA targeted therapy technologies.
(I) introduce Alnylam company and its technology platform?
Alnylam has been established for 20 years and is headquartered in Boston, USA. It is a biomedical company specializing in the development of siRNA drugs. Alnylam has experienced ups and downs in the past 20 years, and now it has finally succeeded-all the five siRNA drugs on the market in the world are developed by the Alnylam (including cooperation), and the company has more pipeline projects in the late clinical stage or preparation stage. It is the only unicorn company in the world that has mastered the technology of the whole process of siRNA drug development. There are a lot of details in the article (Reference 1) written by former CEO John Maraganore and published in Nature Biotechnology in May this year to review the 20-year history of Alnylam, from which we can understand why Alnylam can become the only leader in patent medicine among many siRNA pharmaceutical companies.
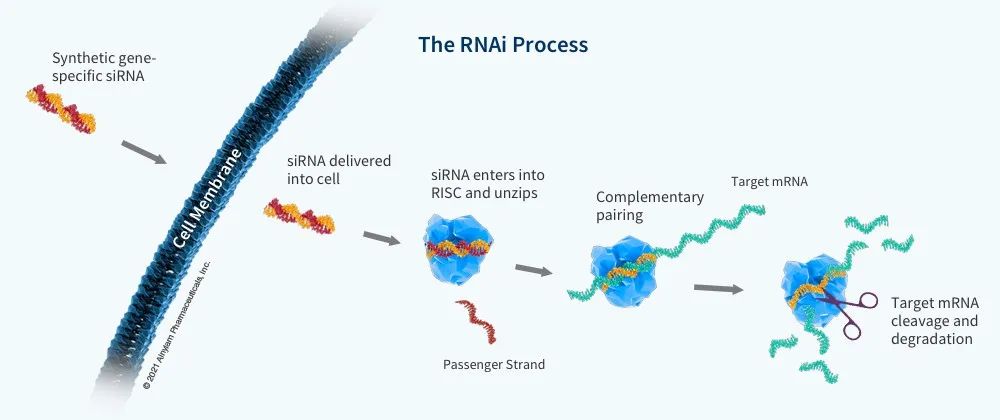
Figure 2. Mechanism of Action of siRNA Drugs (Source: 2)
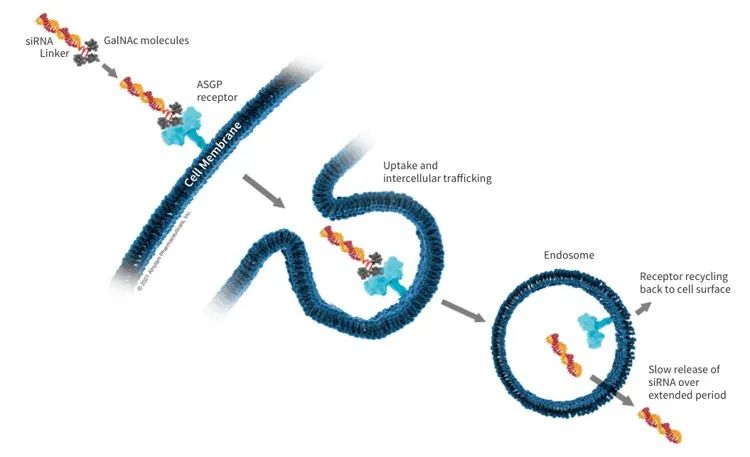
We know that the key to RNA drugs is delivery. Alnylam has been continuously improving and developing siRNA delivery technology. John mentioned that in the first few years, Alnyam spent more than 80% of its research and development expenses on research and development delivery technologies. The company's first generation delivery technology uses LNP as a carrier, such as patisiran. The second generation delivery technology is to couple siRNA with GalNAc. The advantage of GalNAc-coupled siRNA drugs is that they can be highly targeted to hepatocytes, so they can be used to target targets for degradation in the liver. In addition, GalNAc-coupled siRNA can be administered by subcutaneous injection and is extremely stable in the body. A single administration can be effective for half a year or even a year.
Figure 3. siRNA-GalNAc Coupling Delivery Technology (Source: 2)
Alnylam is also developing C16 coupling technology to deliver siRNA to the central nervous system. Polypeptide delivery technology is also under development.
Need to emphasize that any delivery technology is by no means isolated. Finally, if the product is to be successful, there are still many important aspects that need to be perfect, including sequence selection and chemical modification of RNA.
(II)introduce the Alnylam ATTR product pipeline?
Patisiran is the first generation ATTR product, delivered with LNP and requires intravenous injection every three weeks. Approved for listing treatment hATTR-PN in 2018. Judging from today's good news, the patisiran is expected to expand to ATTR-CM indications next year.
Vutrisiran is the second generation ATTR product, which was just approved by FDA for listing therapeutic hATTR-PN in June this year (trade name: Amvuttra). Since the vutrisiran is a GalNAc-siRNA-coupled drug, it is administered subcutaneously and injected every 3 or 6 months. Vutrisiran may also treat ATTR-CM, Hellios-B Phase III is currently underway. In addition, Phase III clinical vutrisiran for a rare eye disease (Staggart's disease, Stargardt disease) is about to begin.
Alnylam also has a third-generation ATTR candidate: ALN-TTRsc04. It was designed through Ikaria technology. ALN-TTRsc04 demonstrated high stability and efficiency in preclinical data. It is possible that, like a vaccine, it only needs one shot a year.
Alnylam evolve and develop products from generation to generation and strive for perfection. There are years of accumulation and even lessons of failure, such as revusiran. The Revusiran is based on the first generation GalNAc platform technology, and the chemical modification is not yet mature.
(III)talk about the significance of successful phase III clinical trials in APOLLO-B?
APOLLO-B Phase III clinical trials are of great significance to the entire ATTR project series. These data prove for the first time that siRNA drugs can successfully treat ATTR-CM. In ATTR-CM indications, siRNA drugs will compete with TTR small molecule stabilizers already on the market (such as Pfizer's VYNDAMAX/tafamidis). Small molecule stabilizers mainly work on mutated TTR, but may be less effective on wild-type TTR in terms of mechanism, and the market for wild-type ATTR is 4-6 times that of hereditary ATTR. siRNA will have a clear effect on both hereditary and mutant TTR, and unlike tafamidis CM treatment approved by FDA, siRNA can treat PN and CM at the same time, thus its application scope is wider.
worth noting that some of the 360 patients enrolled in the phase III APOLLO-B clinical trial were tafamidis users.
APOLLO-B data also greatly strengthened confidence in the success of subsequent TTR products.
(IV)Alnylam have to look forward to in the near future?
We are looking forward to the results of Phase III clinical trials of the next generation of ATTR product vutrisiran in ATTR-CM patients. Vutrisiran also extends to Staggart's disease (Stargardt disease), which is also an exciting new direction. The results of these Phase III clinical trials are expected to come in 2-3 years.
In addition, we are also looking forward to seeing the results of ALN-APP phase I trials in patients with early-onset Alzheimer's disease. This is the first clinical attempt of C16 platform delivery in CNS. We should be able to see preliminary data by the end of this year.
We believe that there are three key factors for Alnylam success: innovative culture, talent first and strategic thinking.
Alnylam encourage innovation. Alnylam has established a "20% time rule" to encourage scientists to use 20% of their time to realize their ideas. The development of C16 coupling and GalNAc coupling technology both originated from the 20% time project. Alnylam also published many research results publicly. The advantage of publishing a paper is that the data is tested by peer review. Of course, the disadvantage is that it is possible for competitors to "learn". However, it seems that Alnylam's pursuit of the true reliability of the data has overwhelmed their concerns about competition.
Alnylam attach great importance to talents. In the course of 20 years, Alnylam and the whole RNAi field have experienced great ups and downs. But Alnylam have never stopped soliciting talents. To succeed, a biomedical company cannot rely solely on one or two innovations, but requires hundreds of innovations, distributed throughout the value chain: target selection, product design, drug development, delivery technology, clinical design, production and CMC, and application approval. This requires every link in the chain to have a team of talents to pursue innovation and create value with all their strength.
Alnylam grasp of strategy is also worth learning. In the early days of the company, Alnylam focused on the research and development of delivery technology. They realized that if the delivery problem is not solved, the siRNA drug market will not be able to expand. In 2010, when major pharmaceutical companies withdrew from RNAi field one after another, Alnylam shifted its strategic focus to clinical development and began to transform from technology platform companies to drug companies.
(VI)can you evaluate the potential and advantages of RNAi drugs?
RNAi drugs can target every gene in the whole genome, while for traditional drugs (small molecules and antibody drugs), only about 4% of gene products can be targeted by listed drugs.
At present, RNAi drugs can silence any gene in the liver. When more delivery technologies are developed, RNAi drugs will have greater potential.
RNAi drugs is in vivo stability and long administration cycle. The difficulty in the treatment of many chronic diseases lies in the low medication compliance of patients. If the frequency of patient administration is greatly reduced, the problem of medication compliance is solved to a large extent.
In addition, RNAi drugs are highly drugged. The success rate of Alnylam in the drug development process is much higher than the industry average.
Compared with gene therapy, RNAi drugs only involve mRNA and do not affect the genome, so they have higher safety.
if we expand our experience in RNAi drug development to all RNA-targeting drugs (the direction we are developing at Schinenkang), we will have more flexibility in targeting genes: RNAi drugs can only down-regulate each RNA, while we can down-regulate, improve or edit a certain RNA.
(VII)RNA therapy technology?
Delivery technology is still a development difficulty. Delivery is not just a single technology, but a system: the choice of conjugates, the design of linkers, the chemical modification of oligonucleotides... There are too many conditions to explore to achieve the right therapeutic index.
In addition, CMC cannot be ignored. It is not difficult to produce a few kilograms of oligonucleotides, but to ensure that each batch of drugs is consistent, with high stable purity, activity, quality and safety, requires a lot of technology and a lot of work. Nearly half of the cases in which the FDA's rejection letter was received were due to certain defects in the CMC. The CMC of RNAi is more complex. At present, only five RNAi drugs developed by Alnylam have been approved for marketing, while other RNAi companies have not yet marketed. There are deep-seated reasons for this phenomenon.
(1)Maraganore, J. (2022) Reflections on Alnylam. Nature Biotechnology, 40, 641-650.
(2)https://www.alnylam.com/
(3)Topline Results from APOLLO-B Phase 3 Study of Patisiran 8/3/2022 Alnylam company presentation.
(4) Other public information
Reprinted from the era of medicine, author: Jin Tao Sha Jian